Case Report 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Review of Recent Outpatient Therapeutic Updates for SARS-Cov2
*Corresponding author:James Zapata, Assistant Professor at California Baptist University and Fellowship Faculty of Emergency Medicine at Arrowhead Regional Medical Center, California, United States.
Received: March 14, 2022; Published: March 30, 2022
DOI: 10.34297/AJBSR.2022.15.002178
Abstract
While great strides have been made within the medical community to combat the spread, mortality, and morbidity of SARS-CoV-2, there remains need for identifying effective therapeutics given the ever-evolving nature of the virus. Given the growing number of variants, it is important that medical providers are up to date on the most recent evidence-based therapeutics for the management of SARS-CoV-2. Fortunately, the progress in antivirals and monoclonal antibodies have demonstrated success as outpatient therapeutics for patients at-risk for poor disease outcomes. This article reviews therapeutics approved for outpatient management of SARS-CoV-2 in conjunction with the recently updated guidelines from the World Health Organization, Infectious Disease Society of America, National Institutes of Health, and United States Food and Drug Administration. Monoclonal antibodies Casirivimab plus Imdevimab and Bamlanivimab plus Etesevimab are no longer recommended as a therapeutic for the Omicron variant. Paxlovid, an antiviral, and Sotrovimab, a monoclonal antibody, are considered the therapy of choice for at-risk patients. Remdesivir is considered a reasonable alternative but has intravenous limitations. Molnupiravir is only indicated should all other approved therapeutics be either unavailable or contraindicated.
Keywords: Molnupiravir; Sotrovimab; Caliciviral; Monoclonal; Glycoprotein
Abbreviations: SARS-Cov-2: Severe Acute Respiratory Syndrome Coronavirus 2; RNA: Ribonucleic Acid; MRNA: Messenger RNA; CYP: Cytochrome P450; MAB: Monoclonal Antibodies; RDRP: RNA-Dependent RNA Polymerase
Introduction
Novel severe acute respiratory syndrome coronavirus 2 (SARSCoV- 2), a single-stranded ribonucleic acid (RNA) virus of the family Coronaviridae, swiftly became a chief global health concern in 2019 [1-3]. Originating from Wuhan, China, SARS-CoV-2 quickly spread throughout the world resulting in a pandemic affecting over 260 million people that has claimed at least an estimated 6 million lives based on excess mortality data [4–6]. Great strides have been made within the scientific and medical community to combat the spread, mortality, and morbidity of SARS-CoV-2, yet new avenues of unmet medical needs persist given the ever-evolving nature of the virus. The Omicron variant is the most recent variant of concern identified by the Word Health Organization (WHO) and has quickly become the dominant strain in many countries [6-7]. Most concerning are the 32 mutations in the spike protein given the current iterations of SARS-CoV-2 messenger RNA vaccines (mRNA) encode the SARSCoV- 2 glycoprotein to infer immunity [6-8]. While the development of novel vaccines to combat SARS-CoV-2 has demonstrated utility in reducing morbidity and mortality as a preventative measure, it is not a perfect remedy. Many individuals, whether due to medica contraindications or vaccine hesitancy, are not vaccinated and are at increased risk for poor outcomes [3-10]. Emerging variants have demonstrated increased transmissibility, morbidity, and mortality -even in fully vaccinated individuals- resulting in increased breakthrough cases [3]. There remains a great unmet need for effective therapeutics.
As the virus has continued to evolve, so has the medical community to meet it. In the early days of the pandemic, many medications were repurposed off-label in efforts to find an effective treatment ranging from various antivirals, immunomodulators, corticosteroids, antibiotics, bradykinin receptor antagonists, antimalarial agents, and angiotensin II receptor antagonists. [11] Identifying an effective therapeutic has been challenging given the rapidly evolving nature of the SARS-CoV-2 and its numerous variants [5]. As the scientific method has run its course, potential effective therapeutics have begun to emerge. In addition to symptomatic management, antivirals and monoclonal antibodies (mAb) have demonstrated utility as therapeutics for SARS-CoV-2. Given the growing number of variants, it is important that medical providers are up to date on the most recent evidence-based modalities and guidelines for the management of SARS-CoV-2. This article reviews therapeutics approved for outpatient management of SARS-CoV-2 in conjunction with the recently updated guidelines from the World Health Organization (WHO), Infectious Disease Society of America (IDSA), National Institutes of Health (NIH), and United States Food and Drug Administration (FDA).
Novel Outpatient Therapeutics for SARS-CoV-2
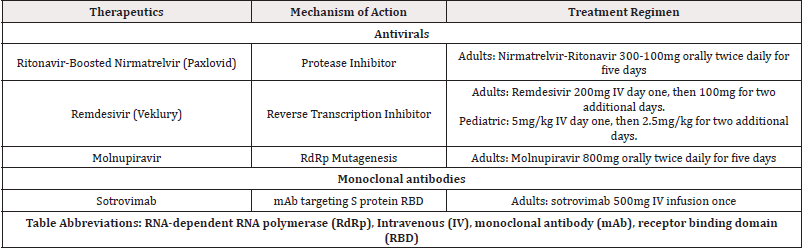
While the advent of novel vaccine modalities has had utility as a preventive measure, there has remained the need for effective therapeutics. Fortunately, the progress in antivirals and mAb therapeutics have demonstrated success as outpatient treatment options for patients at risk for poor disease outcomes. Patients considered to be at-risk, include the elderly, immunocompromised, and those with multiple comorbidities such as cardiovascular, hypertension, diabetes, obesity, and pulmonary diseases [1-13]. (Table 1).
References
- Nhean S, Varela ME, Nguyen YN, Alejandra Juarez, Tuyen Huynh, et al. (2021) COVID-19: A Review of Potential Treatments (Corticosteroids, Remdesivir, Tocilizumab, Bamlanivimab/Etesevimab, and Casirivimab/Imdevimab) and Pharmacological Considerations. J Pharm Pract.
- Frediansyah A, Tiwari R, Sharun K, Dhama K, Harapan H (2021) Antivirals for COVID-19: A critical review. Clin Epidemiol Glob Health 9: 90-98.
- Vasireddy D, Vanaparthy R, Mohan G, Malayala SV, Atluri P (2021) Review of COVID-19 Variants and COVID-19 Vaccine Efficacy: What the Clinician Should Know? J Clin Med Res 13(6); 317-325.
- Simonsen L, Viboud C (2021) A comprehensive look at the COVID-19 pandemic death toll. E Life 10; e71974.
- Roy B, Dhillon JK, Habib N, Pugazhandhi B (2021) Global variants of COVID-19: Current understanding. J Biomed Sci 8(1); 8-11.
- He X, Hong W, Pan X, Lu G, Wei X (2021) SARS-CoV-2 Omicron variant: Characteristics and prevention. MedComm 2(4); 838-845.
- Tian D, Sun Y, Xu H, Ye Q (2022) The emergence and epidemic characteristics of the highly mutated SARS-CoV-2 Omicron variant. J Med Virol.
- Verbeke R, Lentacker I, De Smedt SC, Dewitte H (2021) The dawn of mRNA vaccines: The COVID-19 case. J Controlled Release 333: 511-520.
- Gupta A, Gonzalez-Rojas Y, Juarez E, Manuel Crespo Casal, Jaynier Moya, et al. (2021) Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab. N Engl J Med 385(21); 1941-1950.
- Guidry JPD, Laestadius LI, Vraga EK, Carrie A Miller, Paul B Perrin, et al. (2021) Willingness to get the COVID-19 vaccine with and without emergency use authorization. Am J Infect Control 49(2); 137-142.
- Tarighi P, Eftekhari S, Chizari M, Sabernavaei M, Jafari D, et al. (2021) A review of potential suggested drugs for coronavirus disease (COVID-19) treatment. Eur J Pharmacol 895; 173890.
- (2022) Nonhospitalized Adults: Therapeutic Management. COVID-19 Treatment Guidelines.
- Gallo Marin B, Aghagoli G, Lavine K, et al. (2021) Predictors of COVID-19 severity: A literature review. Rev Med Viro 31(1); e2146.
- (2022) United States Food and Drug Administration. Fact Sheet for Healthcare Providers: Emergency Use Authorization for Plaxlovid.
- Mahase E (2021) Covid-19: Pfizer’s paxlovid is 89% effective in patients at risk of serious illness, company reports. BMJ 375; n2713.
- Wen W, Chen C, Tang J, Chunyi Wang, Mengyun Zhou, et al. (2022) Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19:a meta-analysis. Ann Med 54(1); 516-523.
- Bhimraj A, Morgan R, Shumaker A, Valery Lavergne, Lindsey Baden, et al. (2022) IDSA Guidelines on the Treatment and Management of Patients with COVID-19. Clin Infect Dis; ciaa478.
- (2022) Ritonavir-Boosted Nirmatrelvir (Paxlovid). COVID-19 Treatment Guidelines.
- McDonald EG, Lee TC (2022) Nirmatrelvir-ritonavir for COVID-19. CMAJ 194(6); E218-E218.
- (2022) COVID-19 Treatment Guidelines. Remdesivir.
- (2022) United States Food and Drug Administration. FACT SHEET FOR HEALTHCARE PROVIDERS EMERGENCY USE AUTHORIZATION (EUA) OF VEKLURY.
- Nhean S, Varela ME, Nguyen YN, Alejandra Juarez, Tuyen Huynh, et al. (2021) COVID-19: A Review of Potential Treatments (Corticosteroids, Remdesivir, Tocilizumab, Bamlanivimab/Etesevimab, and Casirivimab/Imdevimab) and Pharmacological Considerations. J Pharm Pract.
- Gottlieb RL, Vaca CE, Paredes R, Jorge Mera, Brandon J Webb, et al. (2022) Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients. N Engl J Med 386(4): 305-315.
- (2022) United States Food and Drug Administration. FACT SHEET FOR HEALTHCARE PROVIDERS: EMERGENCY USE AUTHORIZATION FOR MOLNUPIRAVIR.
- (2022) COVID-19 Treatment Guidelines. Molnupiravir.
- Singh AK, Singh A, Singh R, Misra A (2021) Molnupiravir in COVID-19: A systematic review of literature. Diabetes Metab Syndr Clin Res Rev 15(6): 102329.
- Mahase E (2021) Covid-19: Molnupiravir reduces risk of hospital admission or death by 50% in patients at risk, MSD reports. BMJ 375; n2422.
- (2022) Anti-SARS-CoV-2 Monoclonal Antibodies. COVID-19 Treatment Guidelines.
- (2022) United States Food and Drug Administration. Fact Sheet for Healthcare providers Emergency use authorization (EUA) of sotrovimab.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.